Antimony Sb Has Two Stable Isotopes
4265 123sb 4482 121sb. What are the relative abundances of these isotopes.

Chem1031 Chemistry Textbook Chapter Revision Chem1011 Studocu
These isotopes have almost same natural abundance 5736 sb 121 and 4264 sb 123.

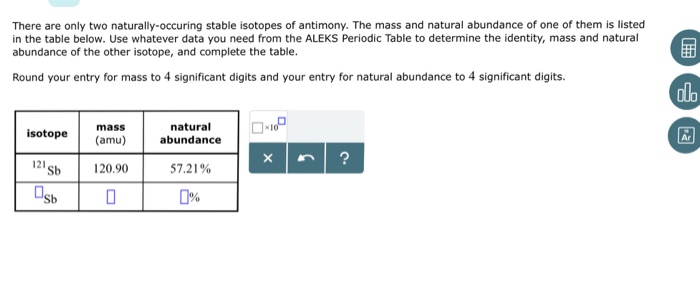
Antimony sb has two stable isotopes. 121 sb with a natural abundance of 5736 and 123 sb with a natural abundance of 4264. There are 35 artificial radioactive isotopes the longest lived of which are 125 sb with a half life of 275856 years. The atomic mass of an element is the weighted average of the masses of its isotopes.
Antimony has 2 naturally occurring isotopes. 4482 123sb 5735 121sb. Antimony sb has two naturally occurring isotopes.
And 126 sb with a half life of 1235 days. It also has 35 radioisotopes of which the longest lived is 125 sb with a half life of 275 years. Using the average atomic mass from the periodic table find the abundance of each isotope.
124 sb with a half life of 602 days. In powdered form antimony is explosive and can lead to spontaneous ignition when exposed to air. It has two stable isotopes antimony 121 and antimony 123.
The atomic mass reported on the periodic table is 7990 determine the mass of bromine 81 the other isotope of bromine. Calculate the percent abundances of these isotopes. Use amu from periodic table and assign x value as decimal abundance for one isotope and 1 x value for other to solve.
Two stable isotopes nearly equal in abundance occur in nature. Antimony 51 sb occurs in two stable isotopes 121 sb and 123 sb. The average atomic mass of antimony is 121757 amu.
Bromine 79 has a mass of 78918 amu and is 5069 abundant. Antimony has 2 stable isotopes 121 sb mass 1209038 and 123 sb mass 1229042 amu. About half of all the antimony produced is reclaimed from scrap lead alloy from old batteries to which antimony had been added to provide hardness.
5518 123sb 4265 121sb. Antimony sb has two stable isotopes with an experimentally determined mass of 120904 amu 121sb and 122904 123sb. The mass of antimony 121 is 120904 amu and the mass of antimony 123 is 122904 amu.
Antimony 121 has a mass of 1209038 u x abundance antimony 123 has a mass of 1229042 u y abundance there are only 2 isotopes for antimony and their percent abundances should add up to 100. One has mass 121 and the other mass 123. Antimony has two stable isotopes.
Bromine has two naturally occurring isotopes.

Solved Question 4 1 Point Both Antimony Sb And Bromin

Chem 1310 Practice Exam Question Bank August 2012 Update Chapter

Unit 2 There Are About 118 Different Known Elements With 88 Of
Antimony Wikiwand
Solved There Are Only Two Naturally Occuring Stable Isoto

Antimony Wikipedia
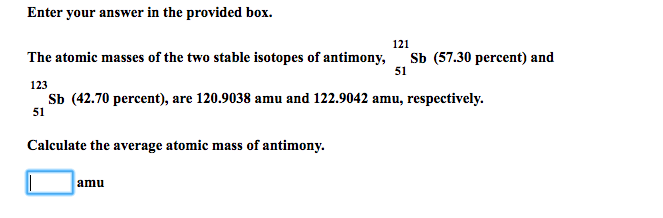
Solved Enter Your Answer In The Provided Box The Atomic

Slides Show

Chapter 2 Atoms And Elements Slideshow And Powerpoint Viewer